BOD troubleshooting
What's causing bias? Contamination? Calibration? Or Seed?
Contamination
- can affect both blanks and GGA.
- tends to be significant effect.
- tends to be high bias (GGA high).
- Must determine whether the source is "food" or "bugs".
Calibration
- mainly affects blanks.
- tends to be small effect.
- can be low or high bias (blanks deplete > 0.2 mg/L or gain > 0.2 mg/L)
Seed
- mainly affects GGA.
- typically an issue of not enough seed.
- tends to be large effect.
- tends to be low bias (GGA low).
Contamination source: Is it from bugs or dirty dishes?
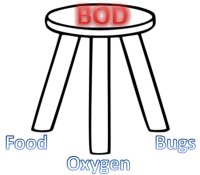
a food source, bugs, and oxygen
for stability.
Just as a stool needs all of its legs to be stable, so too, all "legs of the stool" must be present for a "BOD" to be determined:
You must have bugs, oxygen, and a food source
When it comes to contamination, you can have bacterial contamination, general contamination (food source) or both. The key to successful troubleshooting is to identify the signs which point to whether the problem is related to general contamination, or whether the contamination is due to "bugs".
GGA fails (high bias) but blanks are perfect
The contamination could be "bugs".
- The source could be a bad filter in the DI system (bugs growing within the resins).
- Blanks are likely fine because glassware is clean and there is no "food source" to keep bugs going and expending oxygen.
- GGA fails high due to the extra oxygen consumed by the bugs as they attack the GGA.
The contamination could also be just "dirty glassware", providing a food source.
- Blanks are likely fine because —despite availability of a food source (the "crud")— there is no source of bugs and therefore no oxygen can be used.
- GGA fails high due to the extra oxygen consumed by the bugs as they attack both the GGA and the "crud"
GGA & blanks both fail (high bias) high (blanks deplete 1-2 mg/L)
- The contamination is likely a combination of "dirty glassware", dilution water, and "bugs".
- Blank(s) and GGA fails high because not only is there a food source ("crud") but also there are "bugs" that shouldn't be there.
Excessive depletion in dilution water
Excessive DO depletion in BOD blanks is one of the most common problems that plague all wastewater testing laboratories. Even the best labs occasionally have problems meeting the DO depletion in the BOD blanks (< 0.2 mg/L). The following are the most common causes for excessive depletion in blanks:
- Tubing used for delivery of dilution water is constructed of oxygen-demand leaching material.
- Correct tubing not being used throughout the lab.
- Slime growth in delivery tubing.
- Poor water quality/improperly maintained system.
- Poorly cleaned BOD bottles or dilution water storage container. (Glass is always best!)
- Contamination during aeration.
- Poorly calibrated DO meter.
Solving dilution water quality problems
Try doing a blank using dilution water prepared from a jug of distilled water that's been in the back seat of your vehicle for a week during a particularly hot July!
Bottled water
Generally speaking, avoid "grocery store" distilled water. This water is stored in plastic bottles which can leach oxygen demanding materials. Some analysts have had great luck using "grocery store" distilled water. If you have good luck with a particular brand, don't change. However, be aware that you can't control how the water is stored. If the water sits on a shelf for several months in a hot warehouse, it's going to be a problem. Nothing leaches organic matter from plastic like hot water.
Purchased bottled water - Bottom Line: Use it if it's working for you; but if it isn't try considering alternatives.
The term "absolute difference" is a mathematical expression that means the result cannot be less than zero. A negative result becomes positive. Therefore, the absolute difference of 1-3 is 2 (the negative 2 becomes a positive). One way to look at this is that the smaller of two values is always subtracted from the larger value.
Those that have successfully used "grocery store" generally have a system. Some will go directly to the factory and buy fresh water. They then date the water, store it in a cool, dry place and discard the water after it is a certain age (e.g., expiration date).
Aging Dilution Water
You should not have to age dilution water --as Standard Methods suggests-- if the water is prepared properly. If you have to age to improve quality, you should be concentrating your efforts on improving the preparation process.
Deodorizers and Dilution Water
Avoid using auto-dispensing deodorizers in the laboratory. You may be tempted to use deodorizers because wastewater labs can have a bit of an odor. However, don't use them! They typically use alcohol as a carrier which has a very high BOD.
Dilution water issues - simplest solutions
There are a number of simple solutions to obtaining quality lab reagent water to prepare BOD dilution water.
- Obtain water from another laboratory or vendor: Probably the easiest approach is to obtain water from another laboratory that has a track record of producing consistently high-quality BOD dilution water. It may be easier to haul water once a week than maintaining a lab water system, particularly if you have a small laboratory.
- Purchase water from a source that has proven success: You may also find a commercial source of water that is of consistent quality. If you find a particular brand and supplier that works for you, stick with it! Don't fix it if it isn't broken.
- Buy an all-glass laboratory still and distill your own water: If you only need a limited amount of water (e.g., < 5 gallons per week), consider buying an all glass still. Stills are less costly than other water purification systems and tend to produce quality reagent water providing they are maintained properly.
- Buy a bench-top water RO and polisher combo that will produce ASTM Type I water: Bench-top reverse osmosis (RO)/polisher combination systems that will produce ASTM Type 1 water are also available. These systems work well. However, they tend to be expensive (> $1,000) and require regular maintenance to be effective.
Delivery tubing & disinfection
Disinfect delivery tube weekly, using...
While hydrochloric acid (HCl)can be used for cleaning, it's safer to use a dilute household bleach solution.
- dilute bleach solution: 50mL bleach per 2L of lab reagent water, or
- dilute solution of hydrochloric acid (HCl): 100 mL HCl per 1L lab reagent water
- DO NOT mix acid with bleach! Chlorine gas is produced in this reaction. Even in small quantities, exposure to chlorine gas can be fatal.
- Use reinforced nylon tape around larger bottles for safety.
- Nothing touches water except Teflon or glass
Solving glassware cleanliness problems
- Use a good lab-grade non-phosphate detergent and bleach
- Rinse thoroughly with tap water followed by distilled water
- Allow to dry before storing.
- Always cover glassware and store in a clean, dry place.
- Commercial lab dishwashers with non-phosphate, lab grade detergents and acid rinse solutions (non-phosphate) will also work well.
- Use BOD QC samples (blanks, duplicates and GGA) to evaluate washing effectiveness.
Solving water system issues
Charcoal can become contaminated with bacteria and cause problems as well (in at least one lab's experience).
- Stills Follow manufacturer's recommendations for cleaning and disinfecting.
Simple deionizer systems:
- can work well but can quickly be overgrown with bacteria and mold.
- Can leach organics if not maintained regularly.
- If using simple deionizer system, use nuclear-grade or virgin resin (i.e., Lower grade or "re-used" resins WILL leach organic matter and cause problems).
Troubleshooting consistent high bias in GGA
- If you don't warm the GGA before use, results will be consistently high.
- Seed source selection is critical; if recycling final into primary clarifiers, possibly adding nitrifiers to the seed.
- Compare GGAs seeded with domestic wastewater vs. commercial seed (Polyseed, BOD seed).
- To determine if nitrification is occurring, try adding a nitrification inhibitor.
If nitrification is occurring:
- Select another source (that does not receive final wastewater)
- Use commercial seed
Troubleshooting consistent low results for GGA
- Not enough seed: adjust the amount used until you consistently achieve GGA results in the acceptable range.
- Poor seed quality: try another seed source (mixed liquor; primary; another WWTP; commercially prepared seed) Recall the previous slide showing 2 vs. 4 mL of seed
- GGA too old or contaminated: discard expired or contaminated solutions
- Try another GGA source: Several different types/vendors available (NCL, Fisher, other scientific specialty companies)
Troubleshooting poor precision (samples)
Poor sample precision is best characterized by wide variation among dilutions. You have to remember, however, that BOD is a bioassay technique. This means:
- BOD is inherently less precise than instrumental tests like ammonia and total phosphorus. [Don't expect too much]
- Look into sample measuring technique. [Is the procedure used to pour sample and replicate causing variability?]
- Look for "chunks" that might still be visible. One chunk --even a bug or piece of algae-- that goes into the sample, but a similar chunk does not go into the replicate, can cause replicates to fail [Look closely at the filter residue for the sample and replicate]
- Are you using the appropriate precision measurement tool? [Range works well for influents, but RPD is best for clean effluents]
- More concern with poor precision in final vs. raw [There shouldn't BE "chunks" in effluent!]